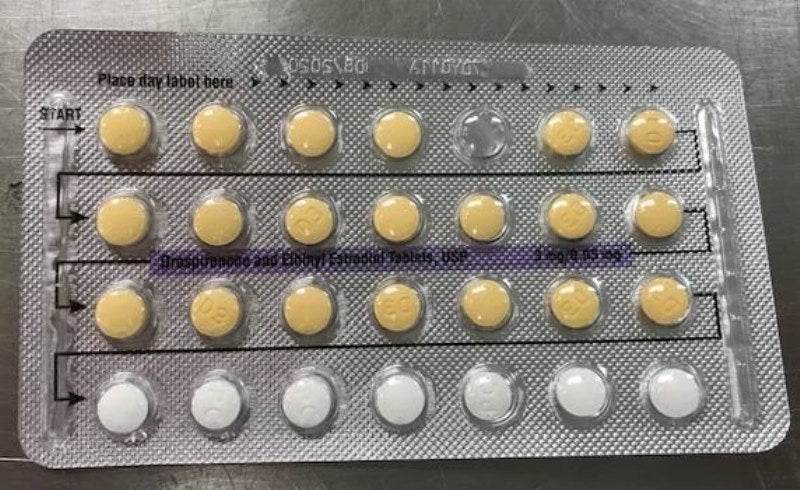
Apotex has issued a voluntary recall of four lots of its Drospirenone and Ethinyl Estradiol tablets, USP 3MG / 0.03MG, due to a US packaging error.
The lots are being recalled as they may possibly contain defective blisters with incorrect tablet arrangements and/or an empty blister pocket.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Due to this packaging error, a patient may not take a dose due to a missing tablet or they may take a placebo instead of an active tablet, which may result in loss of efficacy due to variation in the dosage consumed.
Oman Pharmaceutical Products manufactured the affected product under the subcontract from Germany-based Helm.
Apotex stated: “Drospirenone and Ethinyl Estradiol Tablets, USP, are an estrogen/progestin COC indicated for use by women to prevent pregnancy.
“Drospirenone and ethinyl estradiol tablets (inner carton) consist of 28 film-coated, biconvex tablets in the following order: 21 yellow-colour tablets, each containing 3mg drospirenone (DRSP) and 0.03mg Ethinyl estradiol (EE), and seven placebo white-colour tablets.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company noted that it has not received any reports of adverse events to date.
Apotex has notified its affected wholesalers and distributors via mail. It is also arranging for the return of all the recalled products.
Customers can identify the affected lots through NDC numbers stated on the inner and outer cartons.
Patients with affected lots have been urged to consult their healthcare provider.





