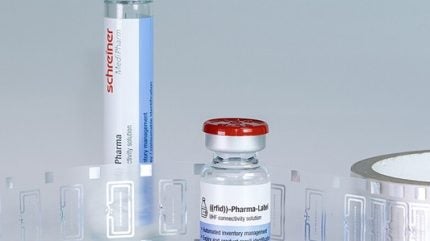
Schreiner MediPharm, in partnership with Bluesight, has developed its smart ‘RFID-Labels’ for enhanced safety and efficiency in the healthcare sector.
The labels are designed to work with Bluesight’s KitCheck systems, offering precise drug tracking and reducing medication errors.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
They are tailored to meet the demands of KitCheck Scanning Stations, ensuring reliable detection of drug containers.
KitCheck has been adopted by 900 North American hospitals, capturing over 325 million units of injectable emergency and anaesthetic drugs.
The RFID-Labels are engineered for durability and can be integrated into pharmaceutical production processes. Their robust structure shields the chip from mechanical damage and functional failures, making them suitable for high-speed labelling systems.
They are also designed to adhere to containers with small radii such as vials and syringes, and offer additional features such as hangers, removable documentation labels, and tamper-evident seals.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe RFID-Labels are automatically scanned at KitCheck Scanning Stations using Bluesight’s tracking software.
This system not only identifies the drug but also captures critical data such as batch numbers, expiration dates, and indications of tampering or previous use. The labels’ digital first-opening indication feature allows for monitoring the integrity of containers at the unit level.
Schreiner MediPharm RFID/NFC solutions senior product manager Sebastian Münscher said: “Standardised and reliable RFID labelling of pharmaceutical products improves workflow, helps reduce medication errors, and enhances patient safety.”
Schreiner MediPharm has also developed a smart packaging solution to track syringes administered in clinical trials.
The new Smart Syringe Box allows digital tracking and temperature monitoring in clinical trials. It integrates technology, safety, and efficiency, which Schreiner says sets new standards for digital applications in clinical research settings.





