How Annex 1 is Changing Leak Detection in the Pharmaceutical Industry

In many ways, this year marks a turning point for pharmaceutical manufacturers. After years of preparation, the industry now faces full enforcement of the updated EU GMP Annex 1 guidelines. For anyone producing sterile pharmaceuticals, Container Closure Integrity Testing (CCIT) has become non-negotiable with the days of bubble testing and dye ingress quickly disappearing.
We have spent nearly two decades perfecting vacuum decay leak detection across industries. But as we enter the pharmaceutical sector more intentionally, we see a unique moment to help both established companies and new market entrants navigate these changing requirements. Truthfully, we see ourselves as a ‘new bee’ in pharma, bringing experience but also listening carefully to the needs of those building their processes for the first time.
The regulatory shift
The revision of EU GMP Annex 1 (effective since August 2023) has triggered a clear shift in expectations. Auditors now expect validated, deterministic, non-destructive leak detection methods that deliver quantitative, reproducible results. The days of subjective pass/fail tests based on visual interpretation are behind us.
At its core, Annex 1 now fully aligns with standards like USP <1207> and ASTM F2338-24. This means methods like vacuum decay, which directly measure pressure changes in sealed containers, are fully recognised as compliant. Probabilistic methods, such as dye ingress and bubble testing, are no longer acceptable for sterile product lines.
The result has been a sharp increase in pharmaceutical companies searching for validated CCIT solutions. Some are replacing outdated methods; others are designing their quality systems from scratch. And many are reaching out for guidance on how to comply without overcomplicating their manufacturing process.
Where we fit in
To meet the growing demand for validated Container Closure Integrity Testing (CCIT), we recently launched two new pharmaceutical-specific machines based on our proven vacuum decay technology.
Our Stationary Leak Tester Pharmaceutical (SLTP) is designed for flexible packaging, including MAP sachets and pouches. It uses a non-destructive vacuum decay method compliant with ASTM F2338 and is ideal for testing multiple units simultaneously. It features an intuitive interface with pass/fail lighting, integrated vacuum and control systems, and is fully 21 CFR Part 11 compliant. The SLTP is already proving invaluable for high-throughput pharma applications where traceable, quantitative testing is essential.
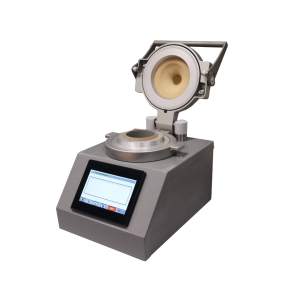
For rigid containers, such as vials, bottles, and capsules, our Container Closure Integrity Tester (CCIT) provides a compact, highly sensitive solution. Also compliant with ASTM F2338 and 21 CFR Part 11, it uses specially designed change parts to accommodate different container formats. Its user-friendly touchscreen and minimal measuring space make it easy to operate while delivering precise, non-invasive results making it ideal for pharma and biotech operations prioritising product integrity and regulatory compliance.
For many pharmaceutical startups and contract manufacturing organisations (CDMOs), this simplicity matters. They need fully compliant solutions that integrate cleanly into existing production environments, without needing excessive training, specialised infrastructure, or costly lifecycle service contracts.
We know that for many of these organisations, leak detection may not be something they have dealt with before. That is why we have built our systems to be highly intuitive, supported by full validation packages, traceable calibration, and straightforward onboarding. We partner with our customers to make compliance achievable.
Supporting the ‘new bees’
One of the most exciting trends we are seeing this year is the rise of smaller pharmaceutical startups, CDMOs, and new sterile fill-finish operations that are proactively building Annex 1 compliance into their operations from the start.
In many ways, their needs match ours: focus on precision, simplicity, and collaboration. They do not need theoretical capabilities but solutions that work, validate, and scale as their business grows. We see this as an opportunity to support fellow “new bees” who are serious about compliance but do not want to overcomplicate their packaging lines.
Compliance without complexity
When I speak to pharma quality managers, the message is often the same: “We don’t just need to pass an audit; we need a system we can trust every day.” That is exactly where deterministic vacuum decay excels.
Our systems generate quantitative test data that is fully audit-ready, compliant with 21 CFR Part 11, easily integrated into electronic batch records, and repeatable across every production run. The result is not just regulatory compliance, but genuine process control.
With Oxipack, leak detection moves from being a one-time qualification hurdle to becoming part of your daily Contamination Control Strategy (CCS). That is where real manufacturing confidence comes from.
Ready to help and listen
As we expand our work in pharmaceuticals, we remain humble about where we are strong and where we are still learning. Our decades of vacuum decay expertise give us confidence, but we are equally committed to listening to what pharma manufacturers need most.
Because in a world where Annex 1 compliance is mandatory, simple, validated, and deterministic solutions are essential.